Life Extension
Therapy
Innovation
Research
|
Advancing Science to Treat Age-Related Diseases and Improve Lifespan.
The Science of Lifespan Limitation

Protein Aggregation
Toxic protein buildup in blood and tissues drives many diseases and shortens lifespan.

Metabolic Disorders
Diabetes and aging disrupt protein folding, forming toxic oligomers and nonfunctional fibrils.
Organ Impact
Protein aggregates damage vessels and vital organs—heart, lungs, pancreas, kidneys, and brain.

Research & Solutions
- The inflammatory stress of aging and many diseases overwhelm and “wear out” the natural repair mechanisms in cells and blood
- These mechanisms lower the stress and disrupt and help dispose of the aggregates.
- At CHEC-PR, short peptides have been isoated and identified that stimulate these normal mechanisms
Toward a further understanding
of the new science of life extension
Exploring protein aggregation, metabolic disorders, and innovative
solutions for age-related diseases.
Toxic Protein Aggregation
The buildup of toxic protein aggregates in the bloodstream and body tissues has become a critical unmet medical challenge. These abnormal protein clusters interfere with normal cellular processes, disrupt organ function, and accelerate the onset of serious health conditions. Their accumulation is closely linked to multiple age-related diseases, contributing to organ damage and significantly reducing both lifespan and overall quality of life.
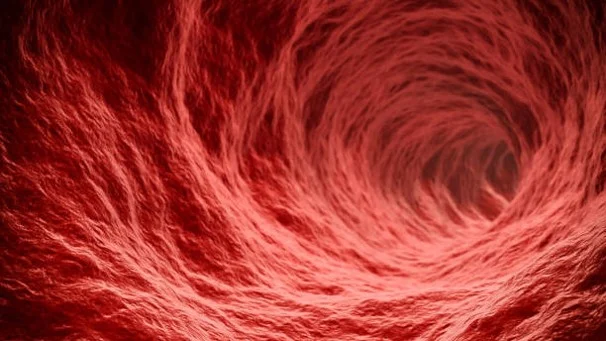
Several endocrine and metabolic disorders, including diabetes and age-related diseases, are characterized by deficits in protein folding, often leading to the formation of toxic oligomers and nonfunctional fibrils and aggregates. In the circulation, these aggregates form from critical blood proteins like albumin, amylin, hemoglobin, and Immunoglobulins. They accumulate on blood vessel walls, in endothelia lining the vessels, and in vital tissues leading to progressive pathological changes in organs like the heart, lungs, pancreas, kidneys and brain.
Quality Control of Protein Structure
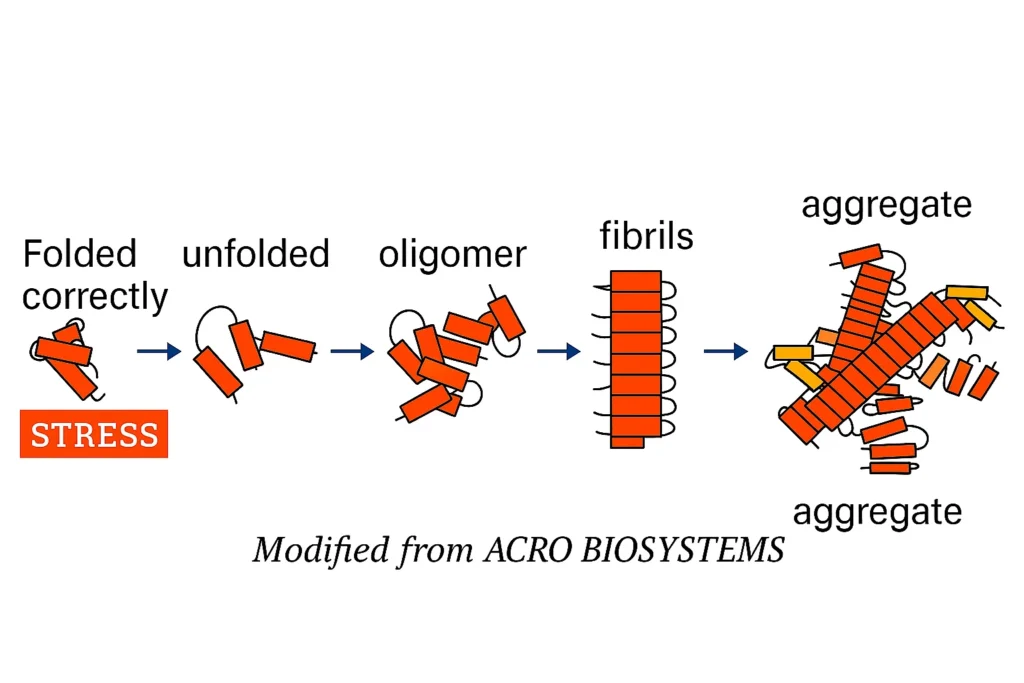
In all cells, proteins are folded correctly by a group of “Chaperones” of which HSP70 is a prominent and important member. If a protein is folded or aggregated beyond repair, HSPs help with its disposal. As we age, and in many age-related diseases, these quality control mechanisms get overloaded and simply wear out.
We documented this same HSP70-mediated QC in human blood plasma and, importantly, that the activity was stimulated by addition of our proprietary peptides1. Other known properties of HSP70, like anti-oxidant and anti-inflammatory activities, were also increased after peptide treatment.
Peptides stimulate HSP70 to attack aggregated, nonfunctional blood proteins.

If protein cannot be repaired, it is dispersed and disposed of.
An important advance occurred in 2011 when Anne Dickinson’s group at Newcastle University reported that peptide sequences from the N-terminus of a human protein (DSEP/Dermcidin), similar or identical to the sequences in out IP portfolio, bound specifically to HSP70 (Stocki, et al., 2011). The DNA coding of the full-length parent protein was identified by our lab and Birgit Schittek at the University of Tubingen.
Current lead compound is cycloSKE7:
Anti-Aggregate, Anti-oxidant, Anti-inflammatory
- High affinity activity (nanomolar to low micromolar) in human plasma with aqueous buffers*
- Derived from human protein = low immunogenicity
- CRO studies on predecessor peptides (similarly contructed) confirm: 1) oral availability and effectiveness with in vivo disease models; 2) No initial stability or safety concerns (see Appendix).
- cycloSKE7: Back to front cyclization increases stability and HSP70 association may prolong in vivo activity (Stocki, et al. 2011)
- Fresh IP: Composition - 12/24; Use, including HSP70 regulation – 2/25
- With MTA in place, peptide samples will be provided for independent testing without obligation.
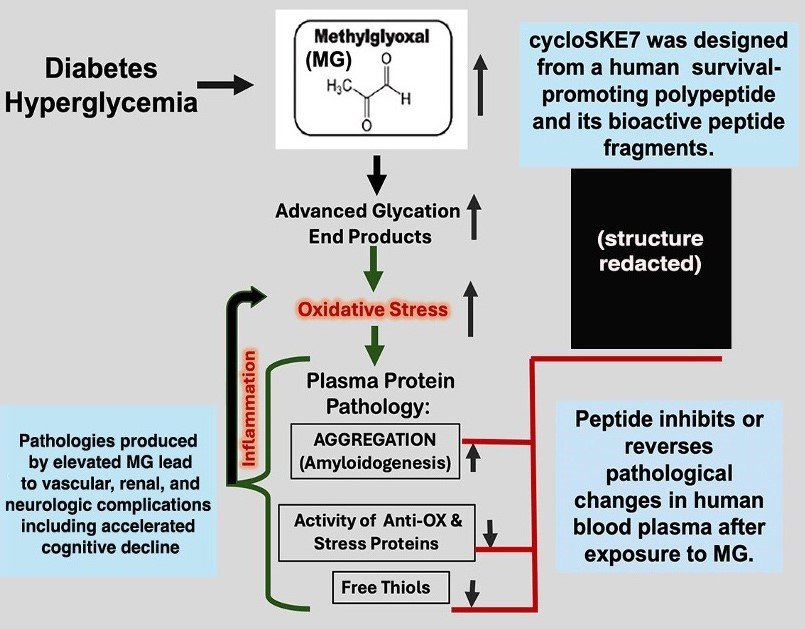
- Data sets will be provided with CDA
Smart Investment Growth
Discover key opportunities to grow your wealth. Navigate through insights,
strategies, and tools designed to maximize returns.
Goal: Complete Clinical Development
Short-Term
Complete IND enabling, regulatory
submission 18 Mo, 3-5M USD
Medium-Term
phased clinical trials, FDA approval 3-5 yrs, Additive funding rounds
Broad applications to a range of aggregate, metabolic, and age-related disorders
Immediate Impact: Markets
- Diabetes (standard & adjunct therapies for complications); Total Diabetes market: United States: 2024 , $379.5 billion, 48 to 64% of expenditures are due to complications from the illness .Global 2024, 1.015 Trillion.
- Life Extension: Global longevity and Anti-Senescence market, $28.13 billion in 2024 to $29.99 billion in 2025 to 38.36 billion by 2029
Connect With Us
Science & Investment:
- Timothy Cunningham, PhD
- Ph. 215 991-8505
- Email. tjc29@drexel.edu
Intellectual Property:
- Robin Stears, Director of IP & Agreements,
- Ph. 215-895-0303
- Email. rls457@drexel.edu
Appendix I
Publication History:
Bioactive DSEP/Dermcidin Peptides
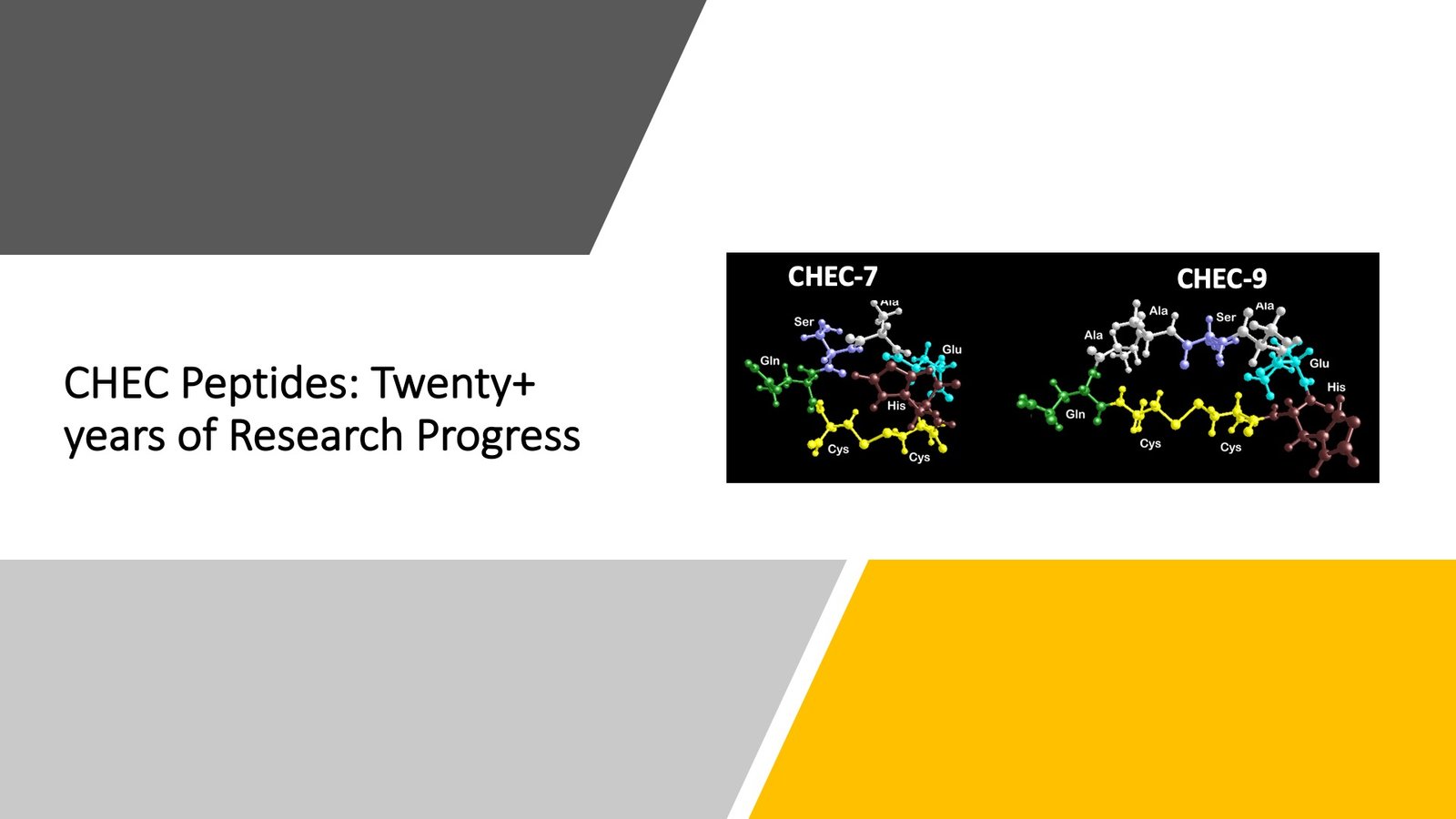










Contract Studies: CHEC Peptides
Studies listed below have been conducted by independent contractors (click to open in new window).
APPENDIX III - Further Reading
Interested in Our Research & Peptides?
Gain access to detailed studies and explore opportunities for collaboration. Fill out the form and our we will get back to you with more information.
Interested in Our Research & Peptides?
Gain access to detailed studies and explore opportunities for collaboration. Fill out the form and our we will get back to you with more information.
